Nasal Spray Recall 2018
Nasal spray recall 2018. Aug 28 2018 Audience. Manukaguard Allercleanse Nasal Spray sold in 13 fluid ounce 40 ml bottles with a batchlot designation of 2010045 and an expiration date of 102023. A Secretaria Nacional do Consumidor Senacom do Ministério da Justiça e da Segurança Pública MJSP encaminhou ofício para o Procon comunicando o recall anunciado pela Laboratórios Ferring Ltda.
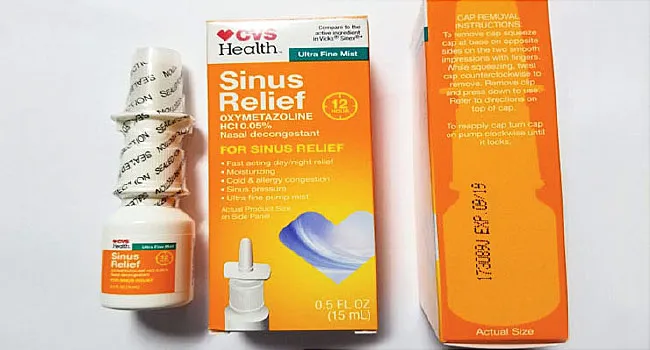
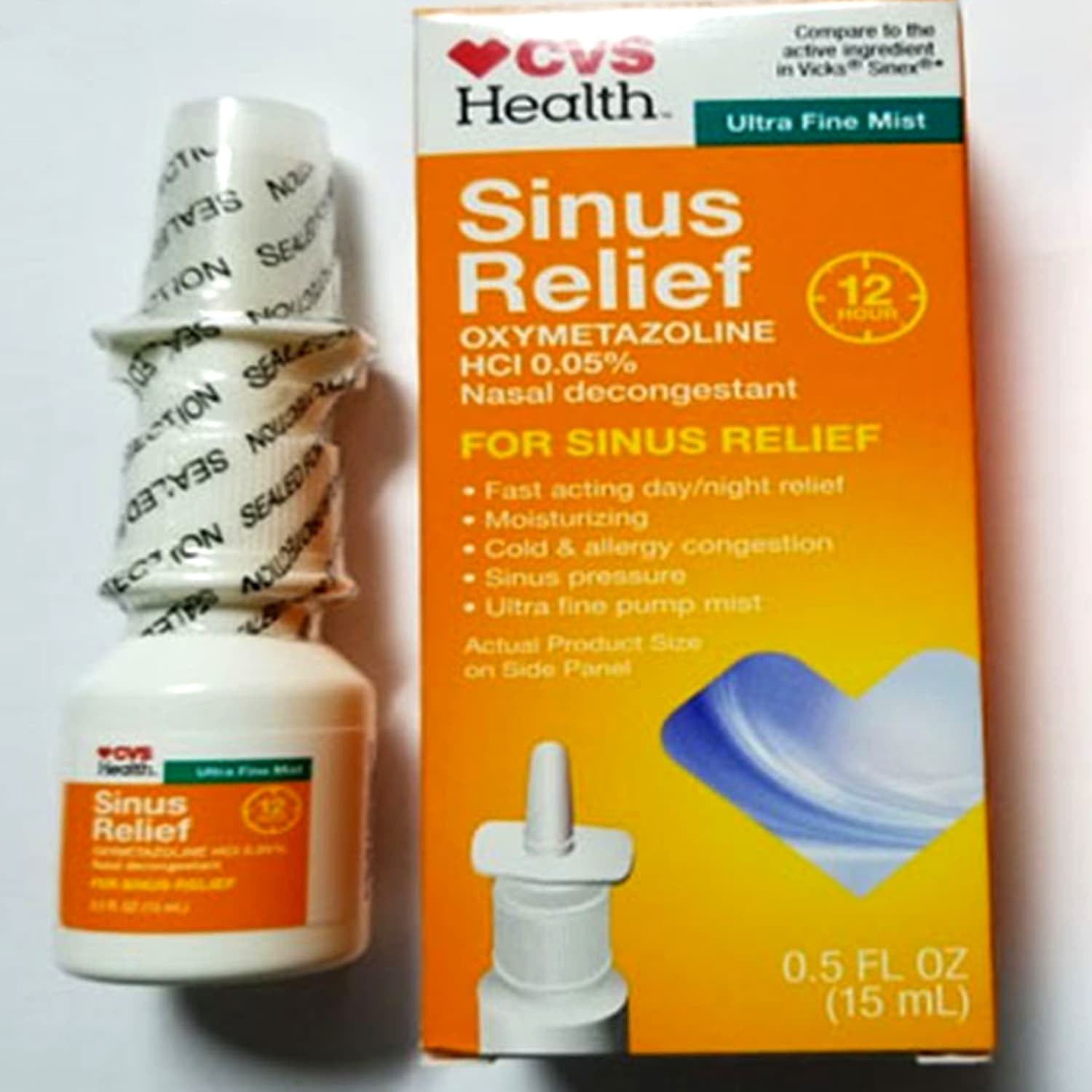
The FDA Alerts below may be specifically about Narcan Nasal Spray or relate to a group or class of drugs which include Narcan Nasal Spray. O Procon-SP orienta os consumidores sobre seus direitos no que diz respeito ao recall do medicamento DDAVP SPRAY NASAL 01 mgml Acetato de Desmopressina. Product Quest Manufacturing Product Quest announced its voluntary recall of Lot 173089J of CVS Health 12 Hour Sinus Relief Nasal Mist due to a finding of microbial contamination identified as Pseudomonas aeruginosa.
Holly Hill FL Product Quest Manufacturing Product Quest is voluntarily recalling Lot 173089J of CVS Health 12 Hour Sinus Relief Nasal Mist a clear colorless liquid to the consumer level. Nasal sprays sold at CVS pharmacies nationwide are being recalled over fears they may contain a microbiological contaminant. O produto que foi convocado pela fabricante é o DDAVP 01 mgmL spray nasal fabricados a partir de 1º de julho de 2018.
There is no known microbial contamination associated with the nasal products and baby oral gels included in the expanded recall. Publix which sells the smaller size announced the recall on its website just as it. Company Announcement Apotex Corp.
Friday Aug 10 2018 at 1027 AM. A Ferring deverá apresentar os esclarecimentos que se fizerem necessários conforme determina o Código de Defesa do Consumidor inclusive com informações claras e precisas sobre os riscos para o consumidor. However the FDA says the recall should be carried out to.
Nasal spray recall issued. Friday June 1st 2018 Fluticasone Propionate Nasal Spray USP 50 mcg per spray 120 Metered Sprays CNW GroupApotex Corp. Narcan Nasal Spray FDA Alerts.
Tens of thousands of CVS nasal sprays recalled because of contamination Updated Jan 30 2019. Consumer Health Professional Pharmacy.
CVS Health 12 Hour Sinus Relief Nasal Mist a nasal decongestant is made by Product Quest ManufacturingThe company initiated the recall over concerns the.
A Secretaria Nacional do Consumidor Senacom do Ministério da Justiça e da Segurança Pública MJSP encaminhou ofício para o Procon comunicando o recall anunciado pela Laboratórios Ferring Ltda. Nationwide nasal spray recall. However the FDA says the recall should be carried out to. Narcan Nasal Spray FDA Alerts. According to the Food Drug Administration Apotex Corp. A Ferring deverá apresentar os esclarecimentos que se fizerem necessários conforme determina o Código de Defesa do Consumidor inclusive com informações claras e precisas sobre os riscos para o consumidor. Nasal sprays sold at CVS pharmacies nationwide are being recalled over fears they may contain a microbiological contaminant. Manukaguard Allercleanse Nasal Spray sold in 13 fluid ounce 40 ml bottles with a batchlot designation of 2010045 and an expiration date of 102023. There is no known microbial contamination associated with the nasal products and baby oral gels included in the expanded recall.
Posted Aug 09 2018 The nasal decongestant product sold at CVS is being recalled. Monday the recalled product was Ocean Saline Nasal Spray in the 15 fluid ounce and 35 fluid ounce bottles. 1 2018 at 821 AM PDT. O produto que foi convocado pela fabricante é o DDAVP 01 mgmL spray nasal fabricados a partir de 1º de julho de 2018. CVS Health 12 Hour Sinus Relief Nasal Mist a nasal decongestant is made by Product Quest ManufacturingThe company initiated the recall over concerns the. Holly Hill FL Product Quest Manufacturing Product Quest is voluntarily recalling Lot 173089J of CVS Health 12 Hour Sinus Relief Nasal Mist a clear colorless liquid. Following is a list of possible medication recalls market withdrawals alerts and warnings.





















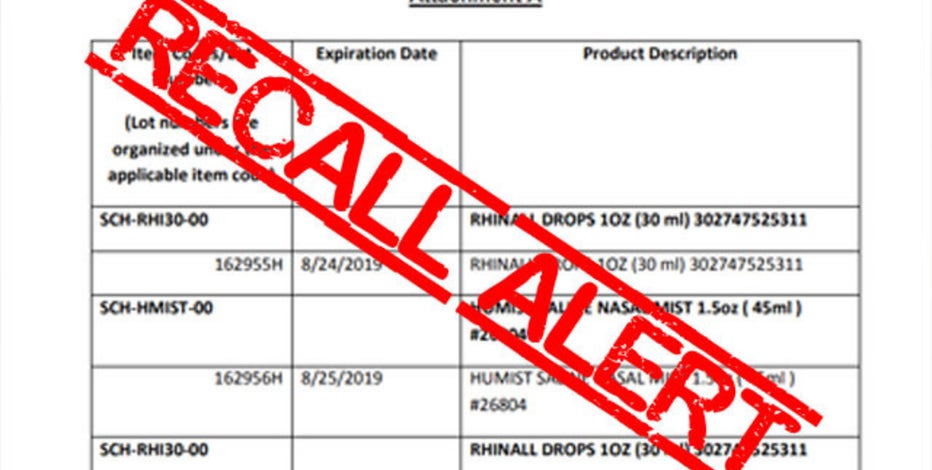

















Post a Comment for "Nasal Spray Recall 2018"